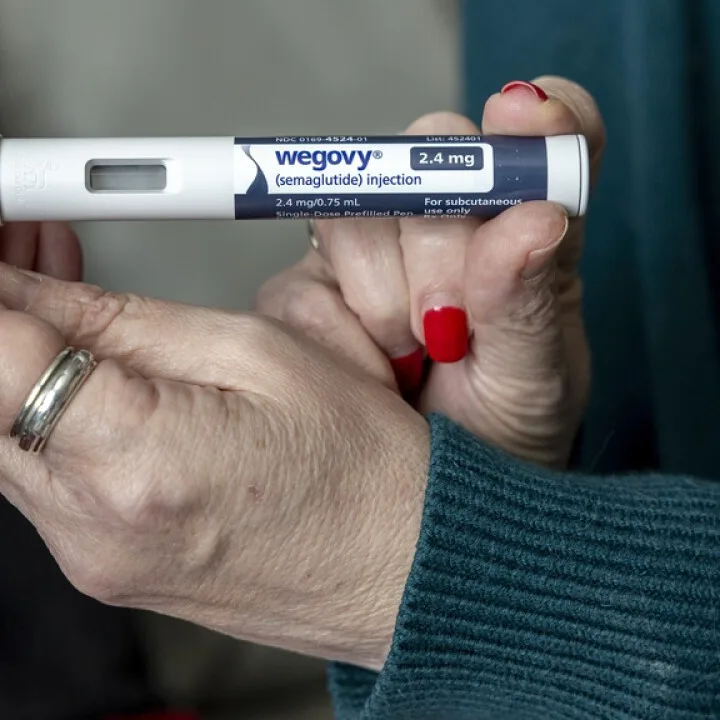
The U.S. Food and Drug Administration (FDA) has officially declared a shortage of two notable diabetes medications produced by Novo Nordisk: Wegovy and Ozempic. This announcement comes after prolonged concerns and reports regarding the availability of semaglutide, the active ingredient in both products, which has gained attention for its use in weight loss and diabetes management. The FDA’s decision marks a significant moment in the ongoing struggle to secure these vital medications for patients. The shortages have been attributed to increased demand outpacing production capabilities. A spokesperson for Novo Nordisk commented, “The patient demand for these medications has significantly increased, and we are working diligently to boost production to meet this need.” In addition, further developments were noted as Hims & Hers saw a decline in their stock shares following an announcement from the FDA, which stated that semaglutide would no longer be listed as a drug in shortage, leading to concerns about the implications for their business model. Principal analyst for Hims & Hers, Dr. Ben Hwang, emphasized in a statement that, “The evolving regulatory landscape means that we need to be adaptable to changes that can affect our operations and market strategies.” This succession of events highlights the dynamic nature of the pharmaceutical supply chain and its direct impact on healthcare providers and patients alike. Furthermore, the ongoing updates from the FDA concerning semaglutide will be closely monitored by industry stakeholders as the situation evolves, ensuring that patient care remains at the forefront amidst these challenges.
FDA Declares Shortage of Novo Nordisk’s Wegovy and Ozempic Drugs: Recent Developments